Is all your product documentation and labeling content compliant with new regulations? Our experts are ready to do a full assessment and let you know if there are any gaps to fill.
With new regulatory requirements just around the corner, it’s important to make sure your entire organization is compliant with the requirements set out in the EU MDR and IVDR. In some areas you may need additional support, and for that we recommend our Life Science consulting services.
How can we help?
- Content translatability assessment – a review of your content through the eyes of a translator, providing direction to your content creators and technical writers on clarity and terminology that has not yet been codified or defined.
- MDR readiness – an evaluation to benchmark translation readiness for MDR and IVDR
- MDR terminology database – access to a terminology database created specifically for the MDR and IVDR regulations
- Linguistic asset review – an assessment of your current localization style guides, glossaries, and translation memory strategies
- Content strategy review – an assessment of your current content strategy to implement MDR and IVDR and use “lessons learned” for the next wave of EU or global regulations
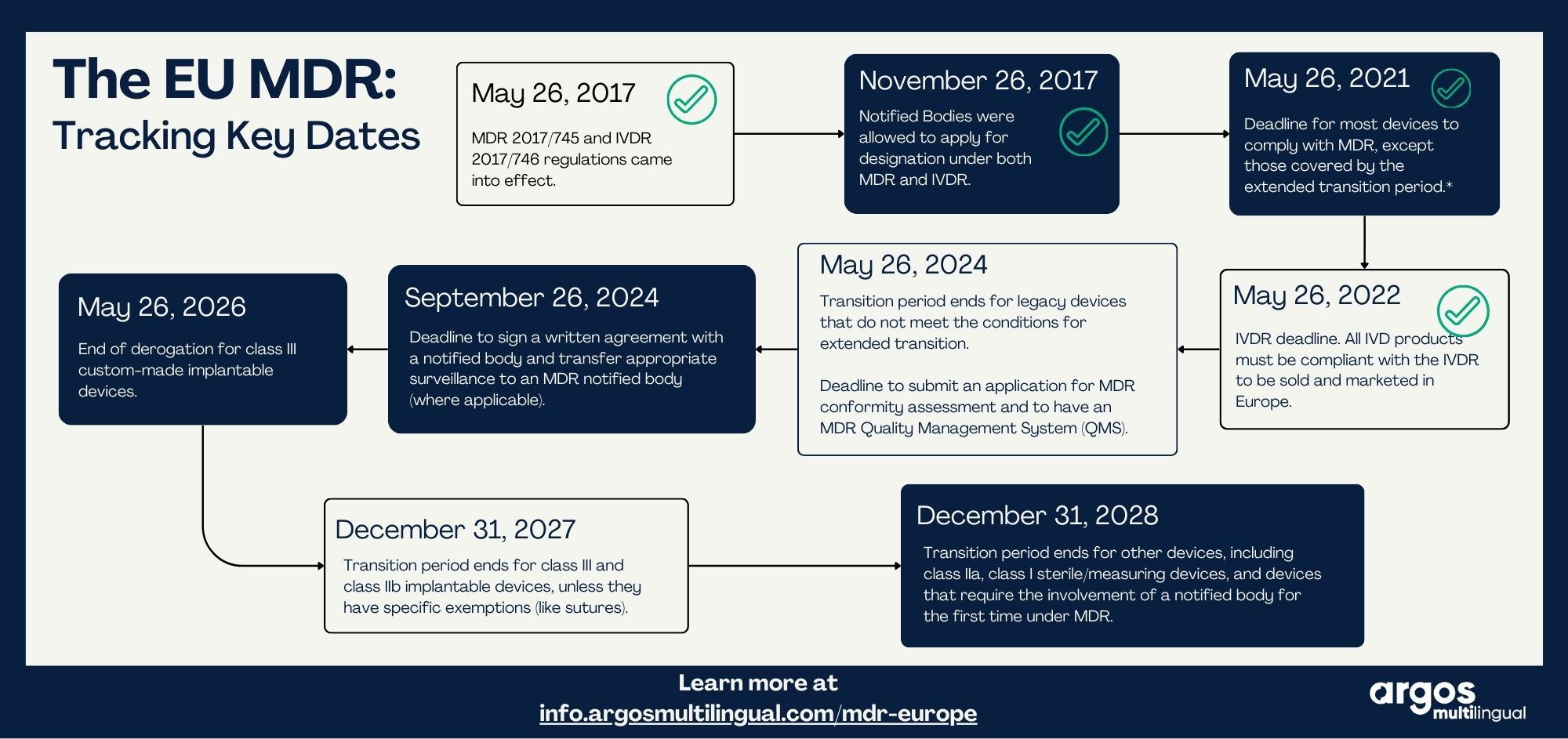
EU MDR Timeline
Deadlines for the EU Medical Device Regulation directive are approaching. Are you IFUs compliant with he new requirements? There are several important dates to remember to comply with the regulation, as outlined in this timeline.
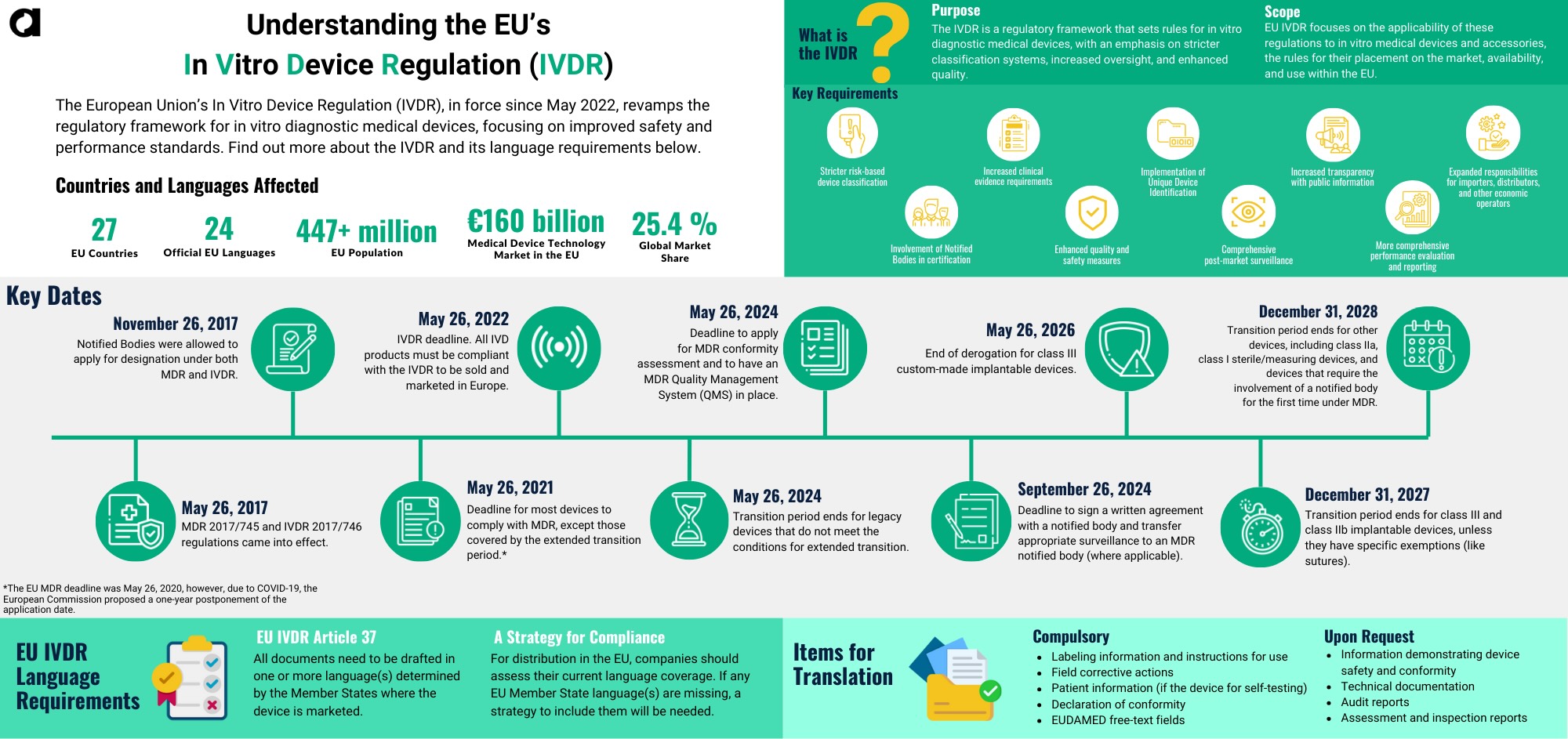
EU IVDR Timeline
The In Vitro Diagnostic Regulation (IVDR) is now in effect. Ensure your organization remains compliant with the latest standards. There are several important dates to remember for the IVDR regulation.
Not sure what the latest updates to regulations are?
We are happy to answer any questions you have and get your project on its way.
Additional services